How Things Melt
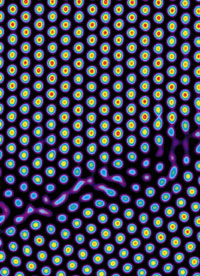 |
| Premelting at a grain boundary within a crystal |
When ice cubes melt, the solid crystals turn to water. It’s pretty simple, right? Not really, says physics professor Arjun Yodh, the James M. Skinner Professor of Science. “Melting is one of the most fundamental phenomena in physics, and yet there are lots of things we don’t understand about it,” he says. “When ice heats up, molecules within the ice acquire more energy and jiggle around more, driving the transition from a solid to a liquid. This is true in part, but reality is richer and more complex.” To look deeper into that complexity, Yodh and his team made “atoms” that were big enough for researchers to see but also sufficiently transparent so they could look at what goes on inside the solid structure. “We created translucent three-dimensional crystals from thermally-responsive colloidal spheres,” explains doctoral student Ahmed Alsayed, which “behave like enormous versions of atoms for the purpose of the experiment.” The scientists observed with video microscopy that “premelting” occurred along the boundaries of imperfections in the orderly structure of crystals, especially where the patterns of the atoms shift. “Premelting was first revealed as an increased movement near defects in the crystal,” Alsayed says. “These motions then spread into the more ordered parts of the crystal.” Yodh notes that understanding this effect could lead to the design of new materials able withstand stresses at higher temperatures.

